- Dr. Ling Peng, Centre Interdisciplinaire de Nanoscience de Marseille, Aix-Marseille University, CNRS, UMR 7325
- Dr. Zvi Hayouka, The Hebrew University of Jerusalem, Rehovot 76100, Israel
- Dr. Mangesh Bhide, University of veterinary medicine and pharmacy, 041 81 Košice, Slovakia
- Dr. Dinesh Dhumal, Centre Interdisciplinaire de Nanoscience de Marseille, Aix-Marseille University, CNRS, UMR 7325
- Dr. Einav Cohan, The Hebrew University of Jerusalem, Rehovot 76100, Israel
- Dr. Zhenbin Lyu, Centre Interdisciplinaire de Nanoscience de Marseille, Aix-Marseille University, CNRS, UMR 7325
- Mr. Bar Maron, The Hebrew University of Jerusalem, Rehovot 76100, Israel
- Dr. Gee W. Lau, University of Illinois at Urbana-Champaign, Urbana, IL 61802
| Keywords | Amphiphilic dendrimers, antibacterial activity, drug-resistant bacteria, drug-resistant biofilm, synergistic effect, targeted drug delivery |
| Current development stage | For Pharmaceutical development: TRL3 – hypothesis testing and initial POC demonstrated in limited # of in-vitro models (in vivo data added in this document) |
| Collaboration Opportunity | Licensing of Technology |
Abstract
Our amphiphilic dendrimers showed potential antibacterial activity against Gram-positive (Staphylococcus aureus, MRSA) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Borrelia burgdorferi and Neisseria meningitidis) bacteria as well as drug-resistant biofilms formed by these pathogens.
Background
Antibacterial resistance has become a global threat, as drug resistance makes bacterial infection increasingly difficult to treat. This pressing public health problem is driving development of new antibacterial agents to overcome drug resistance, which include modification of existing antibiotics, identification of active agents with novel targets against resistant bacteria, and elaboration of antimicrobial peptides with dual antibacterial and immunomodulatory activities, just to name a few. Among these, new antibacterial agents which are substantially different from conventional antibiotics are of particular interest and in urgent need to confront the increasingly alarming antibacterial resistance.
In this context, amphiphilic antibacterial agents are particularly appealing. Among them, amphiphilic dendrimers composed of both hydrophobic entities and hydrophilic dendrons regions are emerging as innovative antibacterial amphiphiles, as they have a distinct dendron composed of core, shell, and surface structure and are synthesized in a step-by-step process for the precise structures. Their amphiphilic nature allows them to self-assemble in aqueous solution into nanostructure and to encapsulate further antibacterial drugs. They have a large number of surface groups that can be functionalized to achieve different properties which makes them a versatile platform for various applications in biomedicine and material science.
Different dendrimers have been studied for their antimicrobial activity. However, active dendrimers are often high-generation molecules bearing charged terminals associated with considerable cytotoxicity.
Recently, small amphiphilic dendrimers composed of hydrophobic entities and hydrophilic dendrons have been explored for antibacterial activity, where their activity was frequently related to dendrimer generation, terminal charge, and hydrophilicity/hydrophobicity balance (Galanakou et al, Biomater. Sci. 2023, DOI: 10.1039/D2BM01878K ; Meyers et al., J Am Chem Soc 2008;130:14444–5; Kannan et al., ACS Appl Bio Mater 2019;2:3212–24; Chen et al., ACS Appl Mater Interfaces 2020). There is still an unmet need for potent and safe antibacterial compounds having novel modes of action and capable of providing effective treatment of bacterial infections.
Our Innovation
We have developed self-assembling amphiphilic poly(amidoamine) (PAMAM) dendrimers exhibiting excellent antibacterial activity. These dendrimers are
- Easy to synthesis
- Non toxic
- Exhibit synergistic combination with clinical antibiotics
- Self-assemble into nanomicelles to deliver drug molecule to infection site
- Nanomicelles can be decorated with targeting moiety such as nanobody and peptide
In addition, some of these dendrimer shows excellent synergistic activity in combination with clinical antibiotics (Doxycycline, Ciprofloxacin) as well as these synergistic combinations can show excellent targeted delivery by decorating the dendrimer nanosystems with nanobodies and/or peptides.
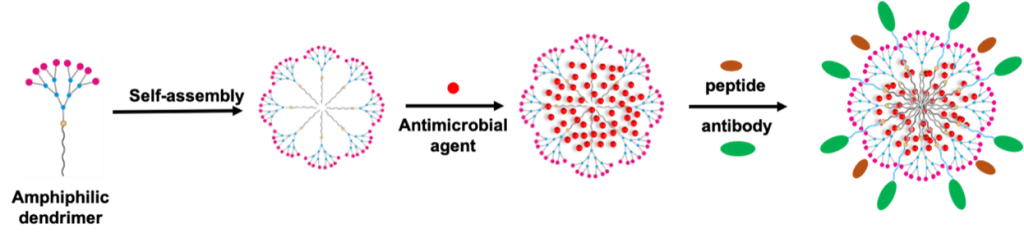
Technology
Our innovative amphiphilic dendrimer consists of an aliphatic hydrophobic chain attached to a hydrophilic PAMAM dendron with scope of abundant variety of surface functionality. These amphiphilic dendrimers form a nanomicelles in the range of 10-30 nm by simple mixing in water. Clinical antibiotics can be encapsulated into the dendrimer nanomicelles for combined activity against bacterial pathogens. In addition, the formed nanomicelles can be further decorated with targeting nanobodies, peptides for bacteria and organ targeting.
Opportunity
The identified active dendrimers are expected to be developed and applied as antibacterial candidates. Impact of this innovation will be very significant on our society which is currently suffering from antimicrobial resistance (AMR). AMR is an urgent global health crisis. In 2019, an estimated 4.95 million deaths were associated with bacterial AMR, a figure likely to exceed 10 million per year by 2050, surpassing cancer-related deaths. This innovation has strength and competitive market for growth.
This technology is ready for licensing.
Patents and Publications
- Dhumal et al., Dynamic self-assembling supramolecular dendrimer nanosystems as potent antibacterial candidates against drug-resistant bacteria and biofilms, Nanoscale, 2022, 14, 9286-9296.
- Peng L, Hayouka Z, Dhumal D, Cohen E, Lyu Z, Bar M. Self-assembling dendrimers and antibacterial uses thereof, US patent application date: May 17, 2022; Application N°: 63/63/214,271. Applicant: The Hebrew University of Jerusalem and CNRS; Filing country: Israel.
